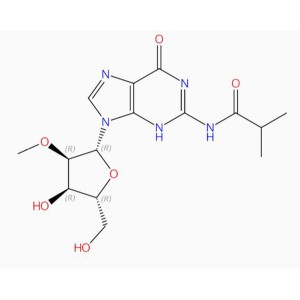
C15H21N5O6 Guanosine, 2′-O-methyl-N-(2-methyl-1-oxopropyl)- (9CI, ACI)
| Sifa Muhimu za Kimwili | Thamani | Hali |
| Uzito wa Masi | 367.36 | - |
| Msongamano (Uliotabiriwa) | 1.68±0.1 g/cm3 | Joto: 20 ° C; Bonyeza: 760 Torr |
| pKa (Iliyotabiriwa) | 9.16±0.20 | Halijoto ya Asidi Zaidi: 25 °C |
TABASAMU za Kisheria O=C1N=C(NC(=O)C(C)C)NC2=C1N=CN2C3OC(CO)C(O)C3OC
Isomeric TABASAMU O(C)[C@H]1[C@H](N2C3=C(N=C2)C(=O)N=C(NC(C(C)C)=O)N3)O[C@H](CO)[C@H]1O
InChI
InChI=1S/C15H21N5O6/c1-6(2)12(23)18-15-17-11-8(13(24)19-15)16-5-20(11)14-10(25-3)9(22)7(4-21)26-14,14-26-14 22H,4H2,1-3H3,(H2,17,18,19,23,24)/t7-,9-,10-,14-/m1/s1
Ufunguo wa InChi
RPULCYXEYODQOG-AKAIJSEGSA-N
2 Majina Mengine ya Dawa hii
2′-O-Methyl-N-(2-methyl-1-oxopropyl)guanosine (ACI);N2-Isobutyryl-2′-O-methylguanosine
Spectra inapatikana
1H NMR
13C NMR
Misa
| Mali zinazopatikana |
| Kibiolojia |
| Kemikali |
| Msongamano |
| Lipinski |
| Kuhusiana na Muundo |
Kibiolojia
| Mali | Thamani | Hali | Chanzo |
| Kipengele cha Mkusanyiko wa Kibiolojia | 1.0 | pH 1; Joto: 25 °C | (1) ACD |
| Kipengele cha Mkusanyiko wa Kibiolojia | 1.0 | pH 2; Joto: 25 °C | (1) ACD |
| Kipengele cha Mkusanyiko wa Kibiolojia | 1.0 | pH 3; Joto: 25 °C | (1) ACD |
| Kipengele cha Mkusanyiko wa Kibiolojia | 1.0 | pH 4; Joto: 25 °C | (1) ACD |
| Kipengele cha Mkusanyiko wa Kibiolojia | 1.0 | pH 5; Joto: 25 °C | (1) ACD |
| Kipengele cha Mkusanyiko wa Kibiolojia | 1.0 | pH 6; Joto: 25 °C | (1) ACD |
| Kipengele cha Mkusanyiko wa Kibiolojia | 1.0 | pH 7; Joto: 25 °C | (1) ACD |
| Kipengele cha Mkusanyiko wa Kibiolojia | 1.0 | pH 8; Joto: 25 °C | (1) ACD |
| Kipengele cha Mkusanyiko wa Kibiolojia | 1.0 | pH 9; Joto: 25 °C | (1) ACD |
| Kipengele cha Mkusanyiko wa Kibiolojia | 1.0 | pH 10; Joto: 25 °C | (1) ACD |
(1) Imekokotolewa kwa kutumia Advanced Kemia Development (ACD/Labs) Programu V11.02 (© 1994-2023 ACD/Labs)
Kemikali
| Mali | Thamani | Hali | Chanzo |
| Koc | 2.12 | pH 1; Joto: 25 °C | (1) ACD |
| Koc | 8.67 | pH 2; Joto: 25 °C | (1) ACD |
| Koc | 12.6 | pH 3; Joto: 25 °C | (1) ACD |
| Koc | 13.3 | pH 4; Joto: 25 °C | (1) ACD |
| Koc | 13.3 | pH 5; Joto: 25 °C | (1) ACD |
| Koc | 13.3 | pH 6; Joto: 25 °C | (1) ACD |
| Koc | 13.2 | pH 7; Joto: 25 °C | (1) ACD |
| Mali | Thamani | Hali | Chanzo |
| Koc | 12.5 | pH 8; Joto: 25 °C | (1) ACD |
| Koc | 7.85 | pH 9; Joto: 25 °C | (1) ACD |
| Koc | 1.76 | pH 10; Joto: 25 °C | (1) ACD |
| logD | -1.26 | pH 1; Joto: 25 °C | (1) ACD |
| logD | -0.65 | pH 2; Joto: 25 °C | (1) ACD |
| logD | -0.49 | pH 3; Joto: 25 °C | (1) ACD |
| logD | -0.47 | pH 4; Joto: 25 °C | (1) ACD |
| logD | -0.46 | pH 5; Joto: 25 °C | (1) ACD |
| logD | -0.46 | pH 6; Joto: 25 °C | (1) ACD |
| logD | -0.47 | pH 7; Joto: 25 °C | (1) ACD |
| logD | -0.49 | pH 8; Joto: 25 °C | (1) ACD |
| logD | -0.69 | pH 9; Joto: 25 °C | (1) ACD |
| logD | -1.34 | pH 10; Joto: 25 °C | (1) ACD |
| logP | -0.464±0.636 | Joto: 25 °C | (1) ACD |
| Umumunyifu wa Ndani wa Misa | 0.55 g/L | Joto: 25 °C | (1) ACD |
| Umumunyifu wa Misa | 3.5 g/L | pH 1; Joto: 25 °C | (1) ACD |
| Umumunyifu wa Misa | 0.84 g/L | pH 2; Joto: 25 °C | (1) ACD |
| Umumunyifu wa Misa | 0.59 g/L | pH 3; Joto: 25 °C | (1) ACD |
| Umumunyifu wa Misa | 0.55 g/L | pH 4; Joto: 25 °C | (1) ACD |
| Umumunyifu wa Misa | 0.55 g/L | pH 5; Joto: 25 °C | (1) ACD |
| Umumunyifu wa Misa | 0.55 g/L | pH 6; Joto: 25 °C | (1) ACD |
| Umumunyifu wa Misa | 0.55 g/L | pH 7; Joto: 25 °C | (1) ACD |
| Umumunyifu wa Misa | 0.59 g/L | pH 8; Joto: 25 °C | (1) ACD |
| Umumunyifu wa Misa | 0.96 g/L | pH 9; Joto: 25 °C | (1) ACD |
| Umumunyifu wa Misa | 4.4 g/L | pH 10; Joto: 25 °C | (1) ACD |
| Umumunyifu wa Misa | 0.55 g/L | Maji yasio na buffered pH 6.00; Joto: 25 °C | (1) ACD |
| Umumunyifu wa Ndani wa Molar | 1.5 x 10-3 mol/L | Joto: 25 °C | (1) ACD |
| Umumunyifu wa Molar | 9.6 x 10-3 mol/L | pH 1; Joto: 25 °C | (1) ACD |
| Umumunyifu wa Molar | 2.3 x 10-3 mol/L | pH 2; Joto: 25 °C | (1) ACD |
| Umumunyifu wa Molar | 1.6 x 10-3 mol/L | pH 3; Joto: 25 °C | (1) ACD |
| Umumunyifu wa Molar | 1.5 x 10-3 mol/L | pH 4; Joto: 25 °C | (1) ACD |
| Umumunyifu wa Molar | 1.5 x 10-3 mol/L | pH 5; Joto: 25 °C | (1) ACD |
| Mali | Thamani | Hali | Chanzo |
| Umumunyifu wa Molar | 1.5 x 10-3 mol/L | pH 6; Joto: 25 °C | (1) ACD |
| Umumunyifu wa Molar | 1.5 x 10-3 mol/L | pH 7; Joto: 25 °C | (1) ACD |
| Umumunyifu wa Molar | 1.6 x 10-3 mol/L | pH 8; Joto: 25 °C | (1) ACD |
| Umumunyifu wa Molar | 2.6 x 10-3 mol/L | pH 9; Joto: 25 °C | (1) ACD |
| Umumunyifu wa Molar | 0.012 mol/L | pH 10; Joto: 25 °C | (1) ACD |
| Umumunyifu wa Molar | 1.5 x 10-3 mol/L | Maji yasio na buffered pH 6.00; Joto: 25 °C | (1) ACD |
| Uzito wa Masi | 367.36 | ||
| pKa | 9.16±0.20 | Halijoto ya Asidi Zaidi: 25 °C | (1) ACD |
| pKa | 1.73±0.10 | Joto la Msingi Zaidi: 25 °C | (1) ACD |
(1) Imekokotolewa kwa kutumia Advanced Kemia Development (ACD/Labs) Programu V11.02 (© 1994-2023 ACD/Labs)
Msongamano
| Mali | Thamani | Hali | Chanzo |
| Msongamano | 1.68±0.1 g/cm3 | Joto: 20 ° C; Bonyeza: 760 Torr | (1) ACD |
| Kiasi cha Molar | 217.6±7.0 cm3/mol | Joto: 20 ° C; Bonyeza: 760 Torr | (1) ACD |
(1) Imekokotolewa kwa kutumia Advanced Kemia Development (ACD/Labs) Programu V11.02 (© 1994-2023 ACD/Labs)
Lipinski
| Mali | Thamani | Hali | Chanzo |
| Vifungo vinavyoweza kuzungushwa kwa Uhuru | 6 | (1) ACD | |
| H Wakubali | 11 | (1) ACD | |
| H Wafadhili | 4 | (1) ACD | |
| H Jumla ya Mfadhili/Mpokeaji | 15 | (1) ACD | |
| logP | -0.464±0.636 | Joto: 25 °C | (1) ACD |
| Uzito wa Masi | 367.36 |
(1) Imekokotolewa kwa kutumia Advanced Kemia Development (ACD/Labs) Programu V11.02 (© 1994-2023 ACD/Labs)
Kuhusiana na Muundo
| Mali | Thamani | Hali | Chanzo |
| Eneo la Uso wa Polar | 147 A2 | (1) ACD | |
(1) Imekokotolewa kwa kutumia Advanced Kemia Development (ACD/Labs) Programu V11.02 (© 1994-2023 ACD/Labs)
Spectra inapatikana
1H NMR
Spectra inapatikana
13C NMR



![C50H60N5O10P Cytidine, N-benzoyl-5′ -O- [bis(4-methoxyphenyl)phenylmethyl]-2′ -O- (2-methoxyethyl)- 5-methyl-, 3′ – [2-cyanoethyl N,N-bis(1-methylethyl) phosphoramidite)](https://cdn.globalso.com/nvchem/C50H60N5O10P-Cytidine-300x300.png)
![C41H39NO6 1-Pyrrolidinecarboxylic acid, 2-[[bis(4-methoxyphenyl)phenylm ethoxy]methyl]-4-hydroxy-, 9H-fluoren-9-ylmethyl ester, (2S,4R)- (9 CI, ACI)](https://cdn.globalso.com/nvchem/C41H39NO6-1-Pyrrolidinecarboxylic-acid-300x300.jpg)
![C45H56N7O9P Guanosine, 5′ -O- [bis(4-methoxyphenyl)phenylmethyl]-2′ -O-methyl- N-(2-methyl-1-oxopropyl)-, 3′ – [2-cyanoethyl N,N-bis(1-methylethyl) phosphoramidite)](https://cdn.globalso.com/nvchem/C45H56N7O9P-300x300.png)
![L-Ornithinamide, L-valyl-N5-(aminocarbonyl)-N-[4-(hydroxymethyl) phenyl]- (9CI, ACI) H335, H319, H315, H302](https://cdn.globalso.com/nvchem/L-Ornithinamide-300x300.jpg)
![C39H46FN4O8P Uridine, 5′ -O- [bis(4-methoxyphenyl)phenylmethyl]-2′ -deoxy-2′ – fluoro-, 3′ – [2-cyanoethyl N,N-bis(1-methylethyl)phosphoramidite] (ACI)](https://cdn.globalso.com/nvchem/C39H46FN4O8P-Uridine-300x300.png)